Nanozymes are man-made nanomaterials that can catalyse biological reactions and display similarities to biological enzymes. There are generally three types of nanozymes: metal-based nanozymes (e.g. Au, Pt, Co), metal oxides or metal sulfide nanozymes, and carbon-based nanozymes (e.g. fullerenes, graphene, carbon dots).
Most nanozymes currently mimic enzymes that catalyse redox reactions, as their rough surface structure is only able to perform the catalysis of relatively simple chemical reactions. The mechanism of their catalysis of redox reactions involve electron transfer between reactants and the nanozyme surface. Surface parameters like size, surface lattice structure and surface composition of nanozymes are likely to have the greatest effect on the catalytic activity of such nanozymes.
But why the interest in nanozymes? Well, nanozymes tend to be more stable over a wider range of conditions, have adjustable activity, and can perform more diverse functions compared to natural enzymes, which are highly selective.
At present, there are two main strategies being used for the improvement of nanozymes, both of which take inspiration from the enzymes of nature.
The first strategy is adding other species into the reaction mixture to enhance the catalytic activity of nanozymes, similar to how the addition of cofactors enhances the activity of biological enzymes. One category of such “cofactors” of nanozymes are metal ions. Certain metal ions like Hg(II) can stabilise the conformation of nanozymes, aid in the transfer of electrons, and reduce the electrostatic repulsion between reactants.
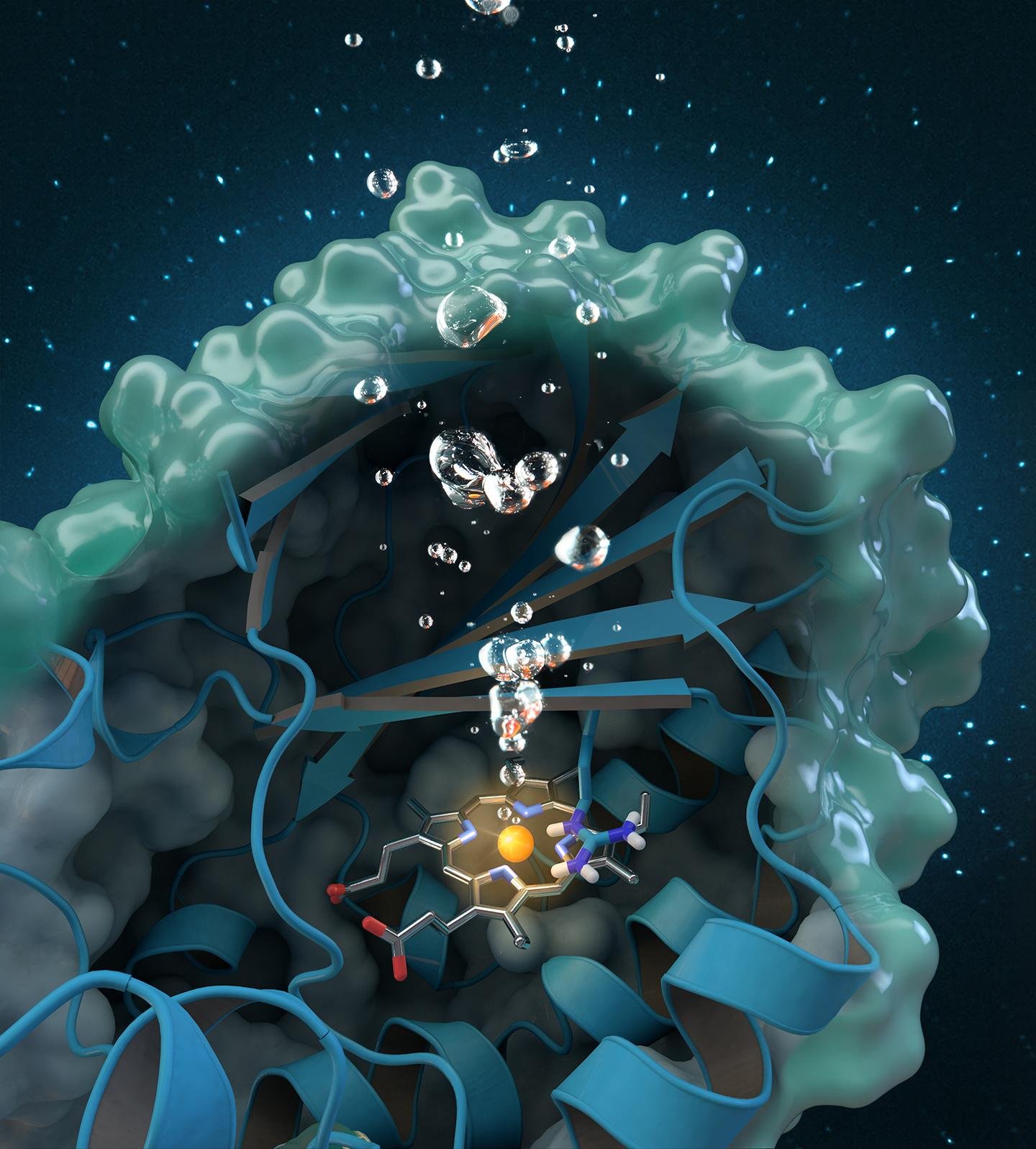
The second strategy is to create finer nanostructures, similar to the active centres of biological enzymes. The first approach to designing such biomimetic nanostructures is to coordinate amino acid residues to the surface of the nanozymes, in a way that is similar to the arrangement and coordination of amino acid residues to the metal centre of metal-containing biological enzymes (metalloenzymes). In biological metalloenzymes, the amino acids in the active sites form attractive interactions with the substrate, keeping the substrate in a specific orientation for reaction, thus being selective to only one substrate. Similarly, the coordination of such amino acid residues to nanozymes not only increases the catalytic activity of the nanozyme by stabilising the nanozyme structure, but also confers increased substrate selectivity.
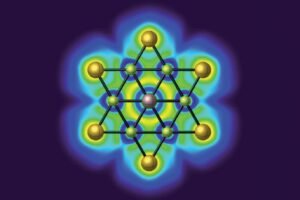
The second approach to designing biomimetic nanozymes considers the fact that in biological metalloenzymes, the metal centre is often a single atom, thus being more efficient than nanozymes which tend to be clusters of metal atoms with only the surface atoms having catalytic properties. In biological metalloenzymes, the single metal atom is anchored within an organic structure by coordinate bonds. Therefore, chemists have synthesised synthetic single-atom nanozymes (SAzymes), where single metal atoms are anchored onto a nanostructure supporting framework.
Analytic techniques have shown impressive similarities in the structure of such SAzymes and biological metalloenzymes. While such SAzymes are still heterogeneous catalysts, they have similar advantages as homogeneous biological enzyme catalysts as the metal atoms in SAzymes are less hindered than in ordinary nanozymes.
An interesting piece of news reported by phys.org is the use of nanozymes as an antimicrobial agent. Such an application is definitely promising, given the problem of antibiotic resistant bacteria following prolonged use of chemical antibiotics. The cerium-oxide based nanozyme, coated with a polymer layer, is able to break down the cell membranes of several pathogenic bacteria. However, due to the fact that nanozymes are often less selective than biological enzymes, the designed nanozyme could potentially damage human cells as well, limiting its applications.
Zhang, R., Fan, K., & Yan, X. (2020). Nanozymes: Created by learning from nature. Science China Life Sciences. doi:10.1007/s11427-019-1570-7
Bangal, P. (2020, June 23). Nanomaterials used as broad-spectrum antimicrobial agents for first time. Retrieved July 02, 2020, from https://phys.org/news/2020-06-nanomaterials-broad-spectrum-antimicrobial-agents.html