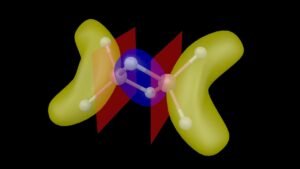
In 2018, Furukawa et al. published an article in Nature Communications Chemistry which aroused great interest in the world of chemists. In his article, he reported the synthesis of an organoselenium cationic species (shown below), which he claimed exhibits double aromaticity.

But first, what does double aromaticity even mean? For analogues of the compounds synthesized by Furukawa et al., double aromaticity can occur through the normal pi aromaticity in the benzene centre, and also sigma aromaticity arising from symmetric molecular orbitals formed from sigma overlap of the orbitals of the substituent atoms, as shown below:

In the synthesized cation, the authors proposed sigma aromaticity to occur in the circle of Se atoms, which they hypothesized to contain 10 electrons (4*2 + 2), thus satisfying the criterion for resonance. But, how can one prove that the synthesized cation exhibits resonance, instead of falling into a structure best described by some other valence bond picture? The authors hypothesized the following possible structures of the synthesized cation:
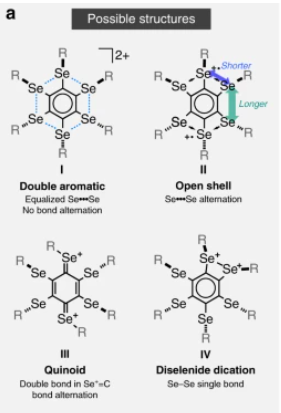
Structure IV above suggests a contribution of [Se-Se]2+ single bonding in the cation. However, the large Se-Se interatomic distance obtained from X-ray diffraction causes the authors to eliminate structure IV as an important resonance contributor.
While the C-Se bond distances was found to be slightly shortened, they are still much longer than C=Se double bonds, and no alternation in the C-C bond lengths of the central benzene ring was observed. Hence, the authors eliminated structure III as an important resonance contributor.
Structure II exists as a triplet state with two unpaired electrons, and would be paramagnetic. Paramagnetism was not observed experimentally, hence the authors also eliminated structure II as an important resonance contributor.
The authors conducted computational chemistry (density functional theory) calculations to evaluate the geometries of the molecular orbitals in the cationic species. The results are shown below:
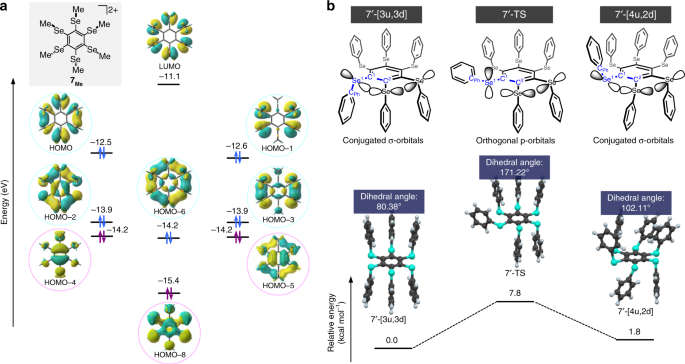
It was observed that the most stable sigma-type molecular orbital, HOMO-6, has no nodes and a symmetric distribution similar to the most stable pi-type molecular orbital in a benzene ring. In addition, the authors identified two stable conformations, 7′-[3u,3d] and 7′-[4u,2d], both allowing for sigma aromaticity in the ring of Se atoms. They found a large activation energy in converting between the two conformations (high energy of the transition state 7′-TS), rationalized by the fact that the Se aromatic interactions need to be broken to convert from one conformation to the other.
In addition, the authors found that the magnetic behaviour of the doubly aromatic cation exhibits magnetic behaviour consistent with double ring current, as it exhibits greater aromatic behaviour in a magnetic field compared to a simple benzene ring.
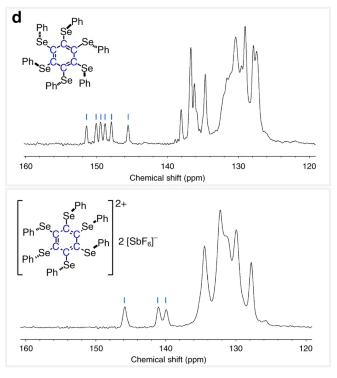
NMR results (shown above) further justifies calling the species “doubly aromatic”. In the neutrally charged molecule where double aromaticity does not exist, 6 signals for the 6 carbon atoms of the central benzene ring was observed. In the cationic species, only 3 peaks were observed with a corresponding upfield shift which means greater electronic shielding, indicative of a distribution of electron density in the ring of Se atoms, and refuting structures placing the 2+ charges on the central benzene ring.
Read the full article at: https://www.nature.com/articles/s42004-018-0057-4