Resonance is a method in valence bond theory (VBT), most commonly used to explain delocalised bonding. In VBT, bonds are assumed to be formed from the overlap of the atomic orbitals of two atoms, and is localised in the region between the two atoms. But, this picture fails when it comes to molecules such as benzene, where the pi bonding is delocalised across the 6-membered carbon ring.
One qualitative way of understanding resonance is that it is taking the average of a few contributing resonance structures, and calling that average the true structure of the molecule. For example, in benzene, we would take the average of the two contributing structures (called resonance structures, or resonance contributors) in order to capture the idea of delocalised bonding:

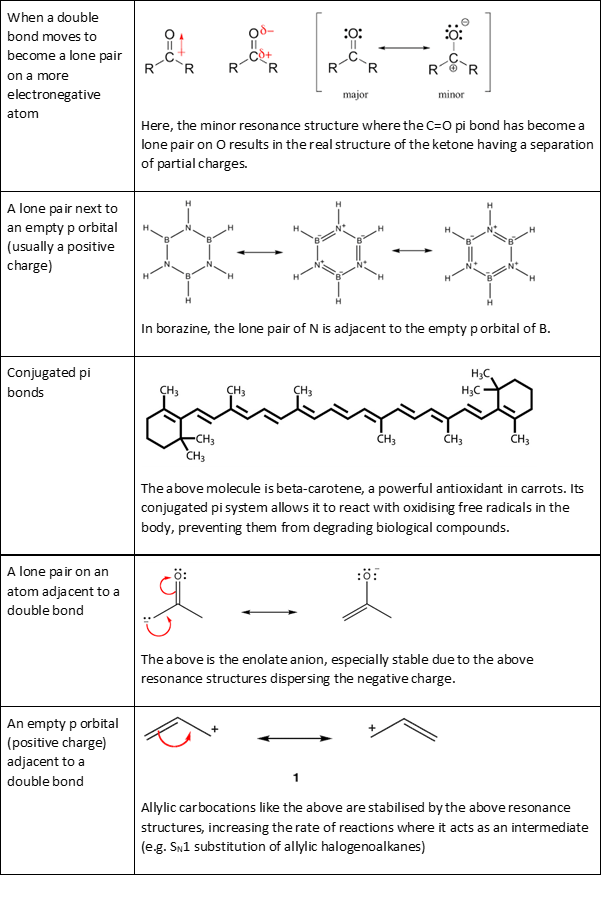
But resonance is also a useful tool even when there is no delocalised bonding. We use resonance whenever the conventional VBT picture of a bond fails, and one example is in the 3-centre-2-electron B-H-B bond in diborane, where two electrons are shared between two internuclear region (instead of one in the usual covalent bond).

In this case, the B-H-B bridge of diborane can be
represented by the average of the following resonance contributors:
Now, when given the Lewis structure of a molecule, how do we
propose the resonance structures which could exist? To propose another possible resonance structure, we “perturb” the original structure according to the following rules:
Resonance structures differ in the position of multiple bonds and nonbonding lone pairs of electrons. The placement of atoms always remains the same, and the placement of single bonds usually stays the same. In other words, from a first structure, we can suggest other resonance structures by breaking and forming pi bonds, but usually not by breaking sigma bonds
Elements in periods 1 and 2 cannot have more than 8 valence electrons.
The reasoning behind these rules is that for a resonance structure to be a significant contributor to the real structure of a molecule, it must have energy similar to (or lower) than the other resonance structures. Usually, breaking sigma bonds raises the energy of the structure by an amount which makes it no longer a significant contributor. Note also that resonance is essentially a picture of electrons in a certain isomer of a compound. We do not shift the position of the atoms relative to each other when drawing resonance structures.
The following table lists cases of resonance which you should be familiar with: